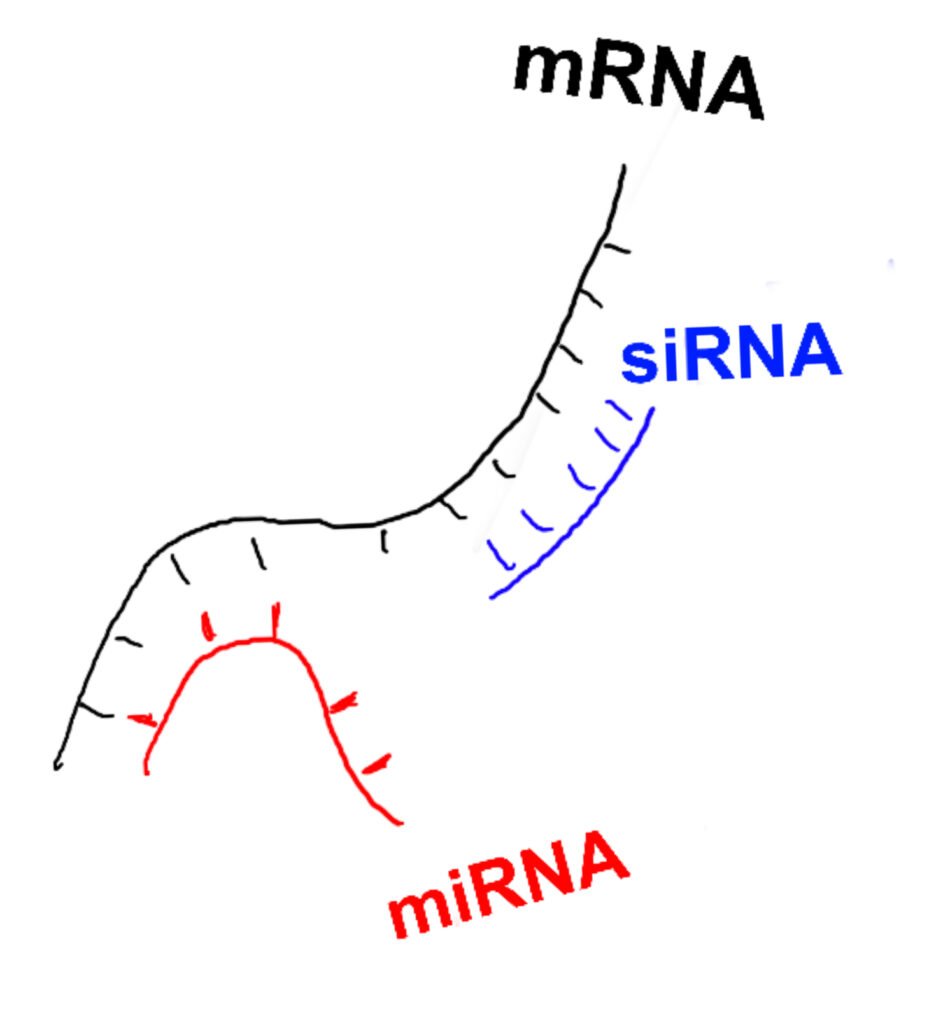
Gene regulation
Gene regulation ensures that, despite all cells having the same genes in all the body cells, cells in specific locations develop unique functions and maintain only those functions. For example, liver cells, kidney cells, and white blood cells all share the same DNA, yet their abilities are expressed differently. This indicates that each cell regulates gene expression in a distinct manner. To achieve this regulation, gene expression is suppressed at the DNA level through various mechanisms.
DNA Methylation
TDNA methylation is an epigenetic mechanism that regulates genes by controlling DNA structures. In this process, a methyl group (-CH₃) is added to cytosine (C) bases, typically at CpG dinucleotides, affecting transcriptional activity. Methylated promoters block transcription factor binding, preventing gene activation. Additionally, Methyl-CpG binding domain (MBD) proteins recruit histone deacetylases (HDACs), leading to chromatin condensation (heterochromatin formation) and transcriptional repression. If these regulatory mechanisms become abnormal, cells may lose their proper function, potentially leading to diseases such as cancer. This explains why, contrary to early expectations that the Human Genome Project would fully unravel the mysteries of the human body, treating diseases like cancer remains extremely challenging even today.
DNA methylation varies in structure across different organisms. For example, when attempting to cut an E. coli plasmid using a restriction enzyme, the presence of methylation on the plasmid may prevent the enzyme from functioning properly. Therefore, it is essential to check the methylation sensitivity of the restriction enzyme before use. If the enzyme is highly sensitive to methylation, it may be necessary to synthesize an unmethylated plasmid via PCR instead of using plasmids synthesized within the cell. There is an example of AatII to show the enzyme blocked by CpG site.

https://enzymefinder.neb.com/#!/name/AatII
Physical modification of DNA
The DNA pin structure is an important mechanism in regulating gene expression as part of chromatin remodeling. DNA forms loop structures by binding with proteins (e.g., CTCF, Cohesin), bringing specific gene regions (such as promoters, enhancers, and silencers) into physical proximity. This is a key component of 3D genome organization, facilitating interactions between enhancers and promoters to activate transcription or, alternatively, forming loops that block transcription factor access, leading to transcriptional repression. Additionally, DNA is divided into structural units called Topologically Associating Domains (TADs), within which genes interact and are expressed. CTCF and Cohesin play crucial roles in loop formation and maintenance, regulating gene expression, while the Mediator Complex promotes the interaction between enhancers and promoters. Increased methylation can hinder the binding of proteins like CTCF, altering the DNA loop structure, which can break the loop between specific enhancers and promoters, leading to decreased gene expression. Conversely, decreased methylation can allow the formation of previously blocked loops, resulting in increased gene expression.